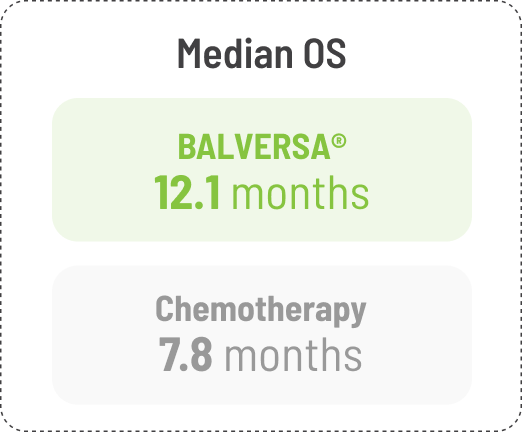
BALVERSA® provided superior OS benefit vs chemotherapy1
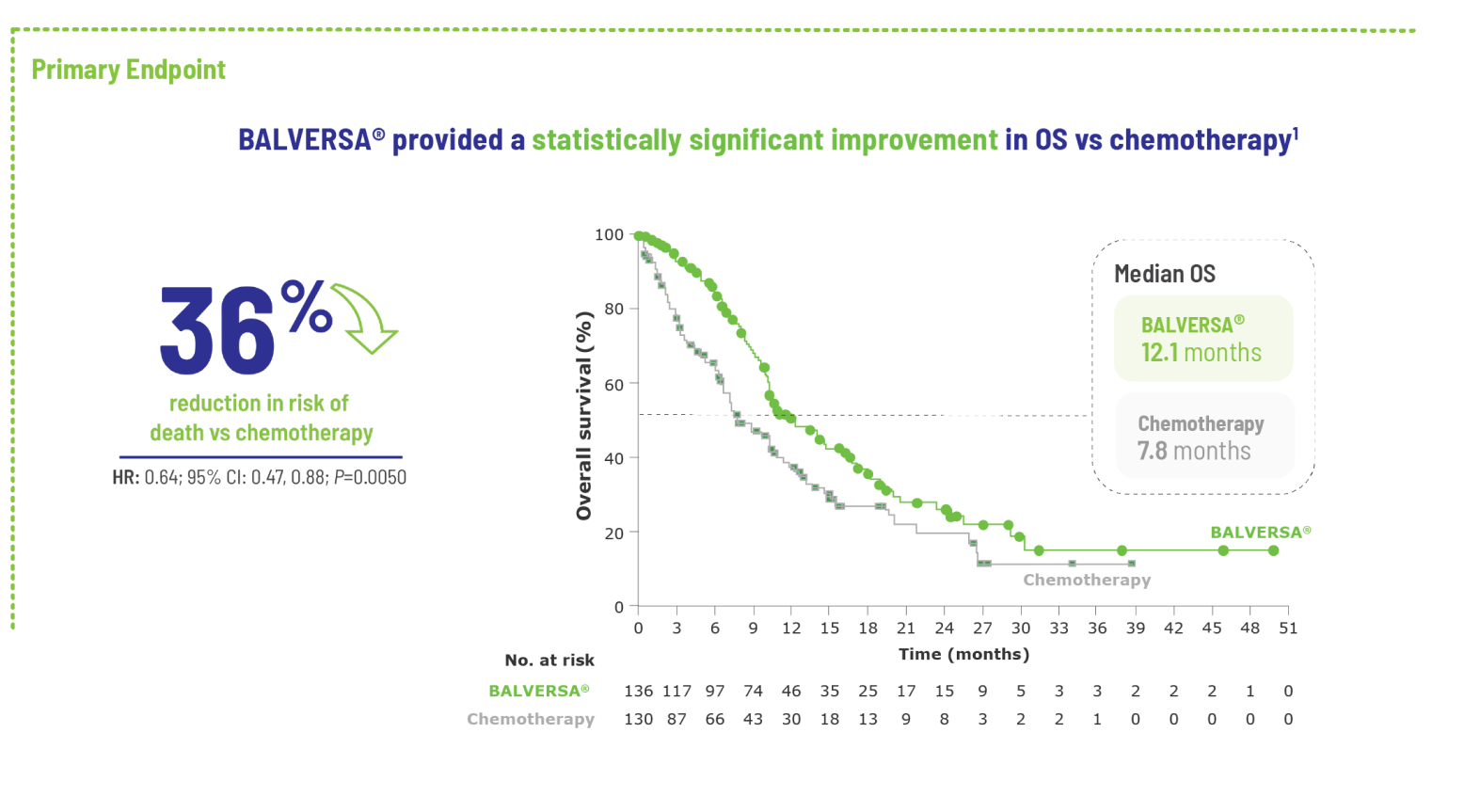
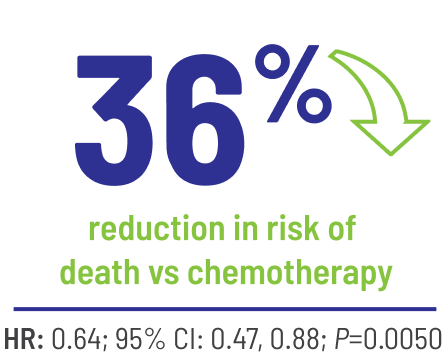
BALVERSA® significantly reduced the risk of death by 36% vs chemotherapy1
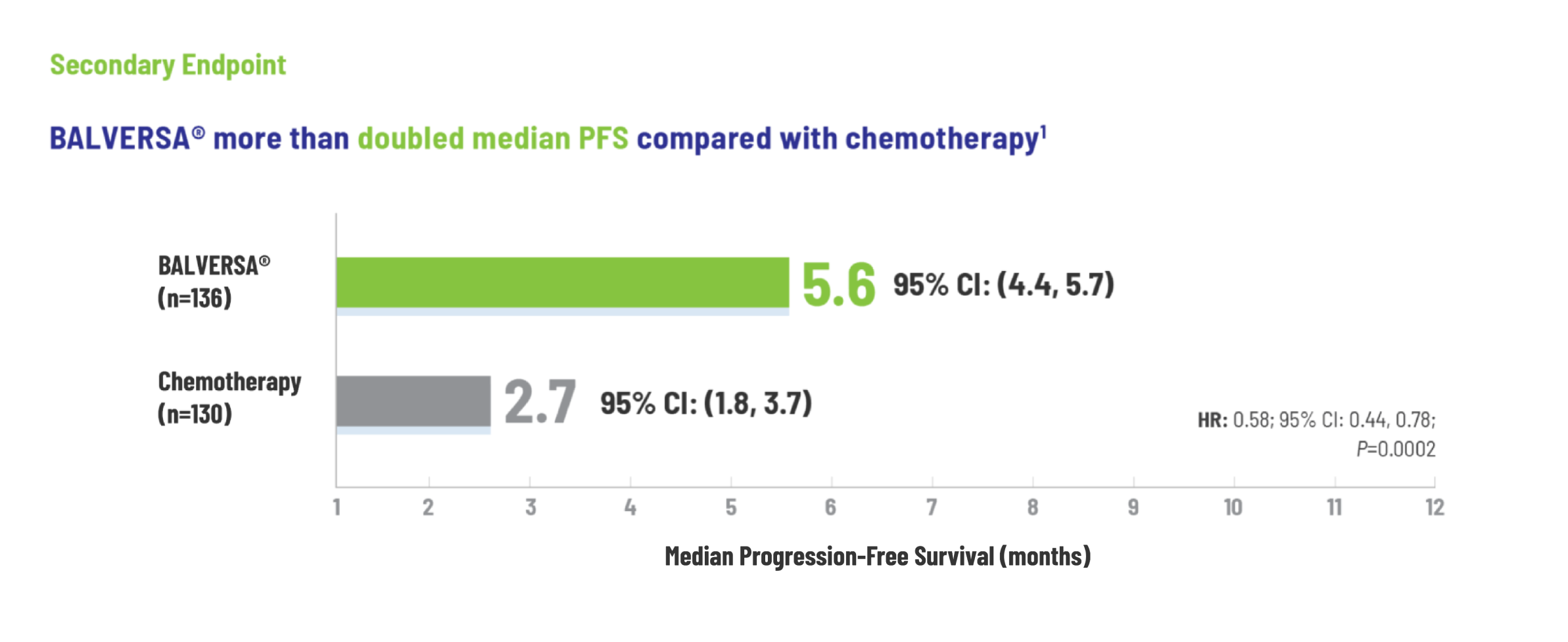
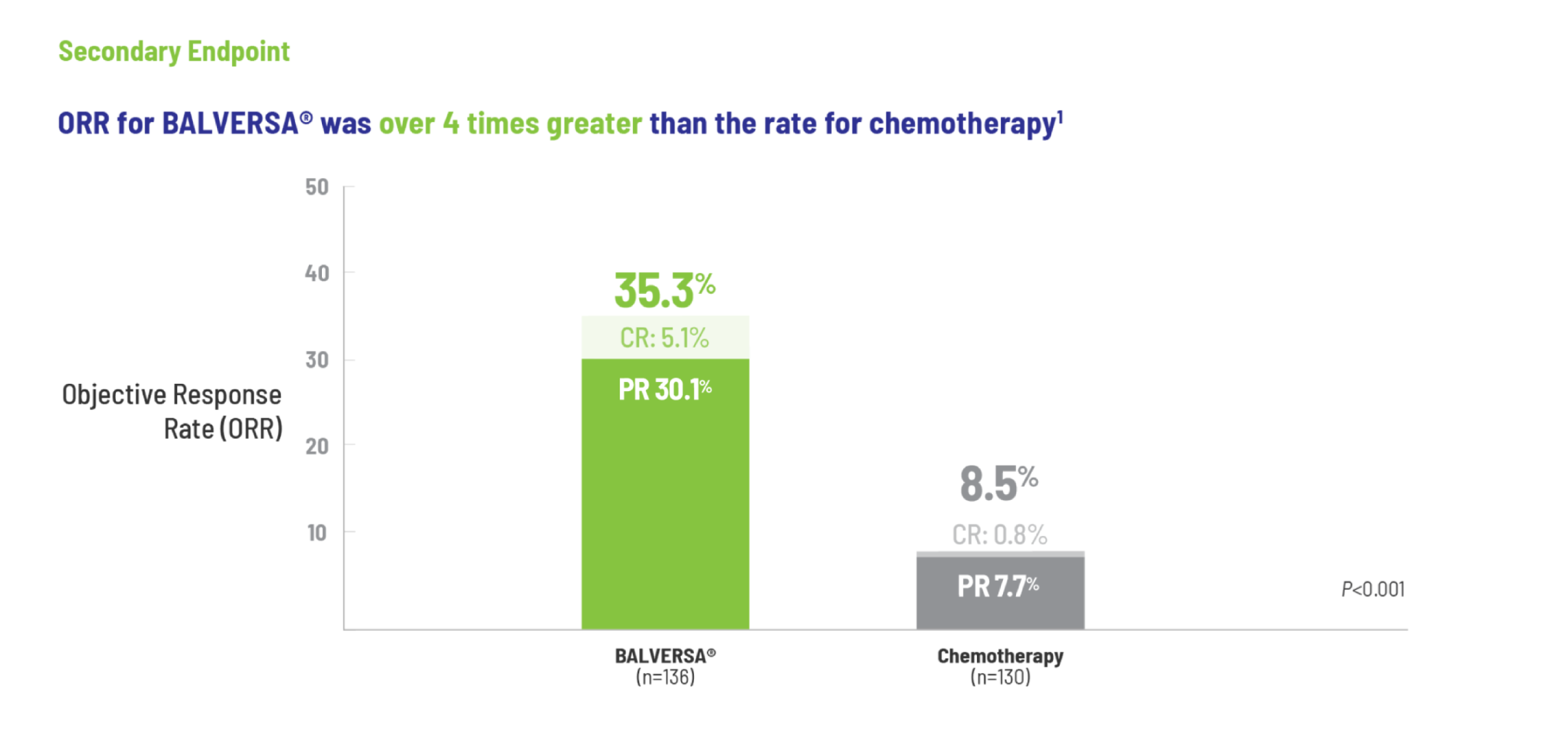
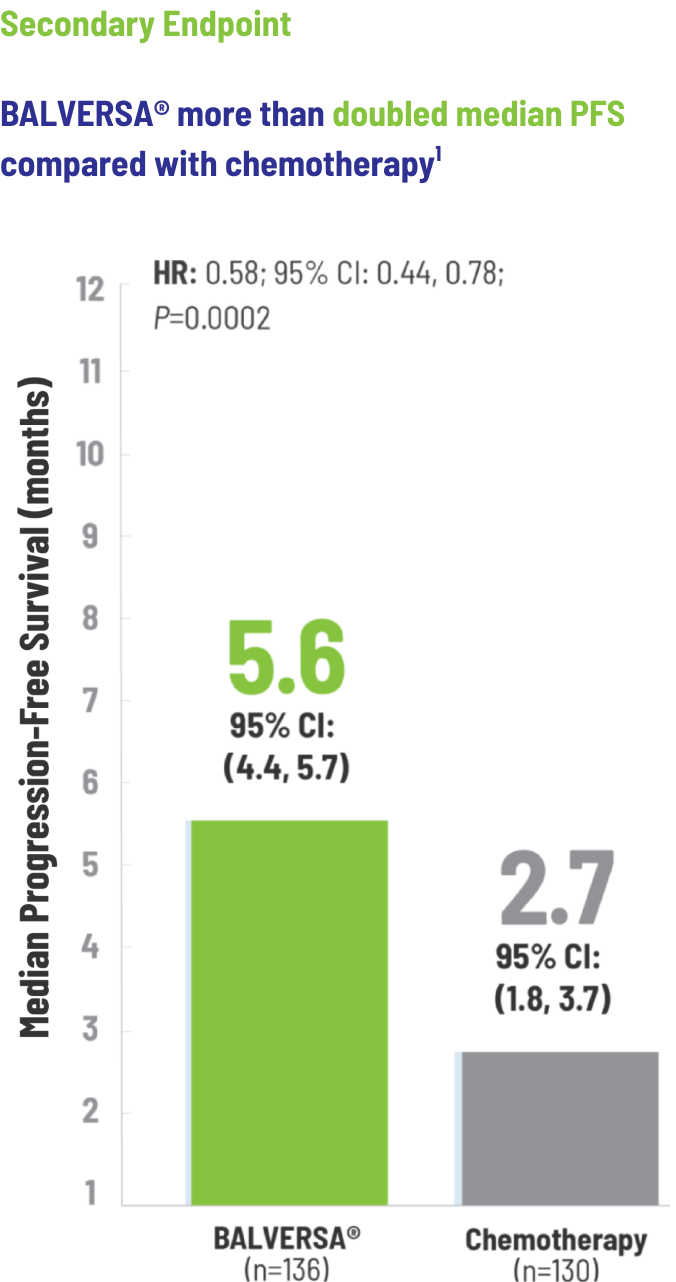
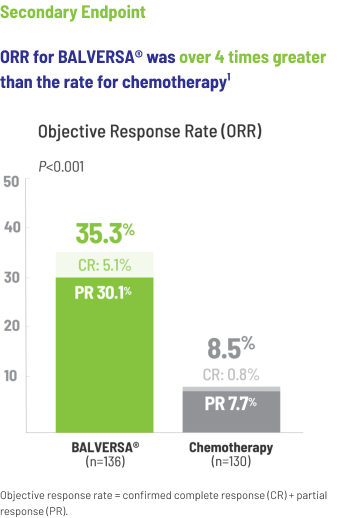
The efficacy of BALVERSA® was studied in a phase 3, randomized, open-label, multicenter study. There were 266 patients eligible for the efficacy analysis; these were patients with locally advanced or metastatic urothelial carcinoma who had had at least 1 prior anti-PD-(L)-1 regimen. The primary efficacy endpoint was overall survival (OS). Progression-free survival (PFS), objective response rate (ORR = complete response [CR] + partial response [PR]) and duration of response (DoR) were included as secondary efficacy endpoints.
Cohort 1
Objective response rate = confirmed complete response (CR) + partial response (PR).
Cohort 2
The study did not meet its major efficacy outcome measure for superiority of OS at the pre-specified final analysis. The OS HR was 1.18 (95% CI: 0.92, 1.51; P=0.18), median 10.9 (95% CI: 9.2, 12.6) months for BALVERSA® vs 11.1 (95% CI: 9.7, 13.6) months for pembrolizumab.
CI = confidence interval; FGFR = fibroblast growth factor receptor; HR = hazard ratio; KM = Kaplan-Meier; OS = overall survival; PD-L1/PD-1 =
programmed death ligand 1/programmed cell death protein 1.
Reference
1. BALVERSA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.