Navigate to a 2L treatment decision with early molecular testing1
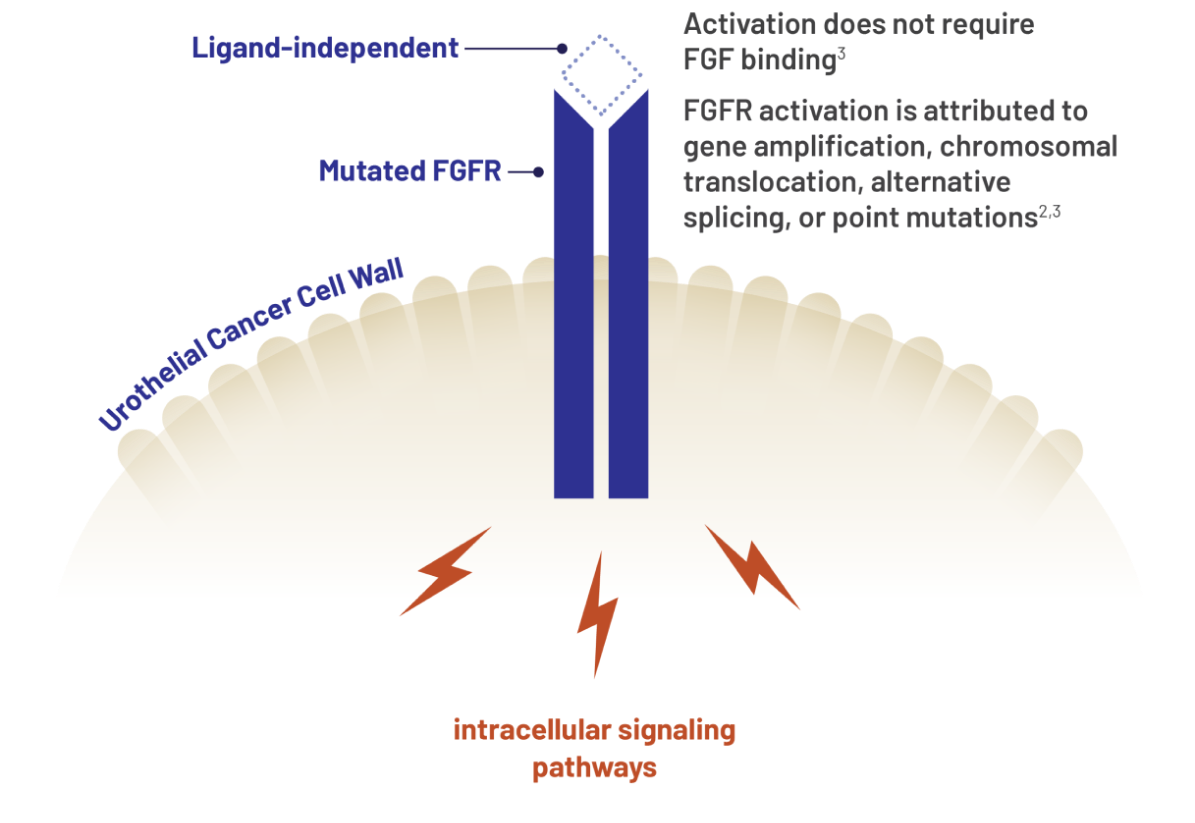
Genetic FGFR alterations/mutations are believed to influence urothelial carcinoma cell growth and proliferation1-3
~20% OF PATIENTS WITH MIBC HAVE
FGFR3 ALTERATIONS/MUTATIONS1,4,5
Recent interest in the development of targeted therapies for advanced urothelial carcinoma is validated by data establishing that these cancers can harbor genetic alterations. Some of the most common alterations occur in the FGFR genes, particularly FGFR3.4,6,7
Genetic FGFR mutations may cause constitutive signaling2,3
Test for FGFR alterations/mutations to identify patients who may be eligible for BALVERSA®
Learn more about an FDA-approved companion diagnostic test at QIAGEN.com
An FDA-approved companion diagnostic test helps identify patients whose tumors have certain FGFR genetic alterations.8 The test detects certain clinically actionable FGFR genetic alterations from formalin-fixed paraffin-embedded urothelial tumor tissue.9
The approved companion diagnostic is available in various national and regional molecular pathology testing labs.
- Find a testing lab located near you
2L = second-line; FDA = U.S. Food and Drug Administration; FGF = fibroblast growth factor; FGFR = fibroblast growth factor receptor; MIBC = muscle-invasive bladder cancer.
References
1. di Martino E, Tomlinson DC, Williams SV, Knowles MA. A place for precision medicine in bladder cancer: targeting the FGFRs. Future Oncol. 2016;12(19):2243-2263. 2. Perera TPS, Jovcheva E, Mevellec L, et al. Discovery and pharmacological characterization of JNJ-42756493 (erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol Cancer Ther. 2017;16(6):1010-1020. 3. Karkera JD, Cardona GM, Bell K, et al. Oncogenic characterization and pharmacologic sensitivity of activating fibroblast growth factor receptor (FGFR) genetic alterations to the selective FGFR inhibitor erdafitinib. Mol Cancer Ther. 2017;16(8):1717-1726. 4. Helsten T, Elkin S, Arthur E, et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2015;22(1):259-267. 5. Tomlinson DC, Baldo O, Harnden P, et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213(1):91-98. 6. Ross JS, Wang K, Al-Rohil RN, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Modern Pathol. 2014;27(2):271-280. 7. Ross JS, Wang K, Khaira D, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122(5):702-711. 8. US Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Accessed January 17, 2023. https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools 9. therascreen® FGFR RGQ RT-PCR Kit, Instructions for Use (Handbook). HB-2561-001, version 1, April 2019. Germantown, MD: QIAGEN N.V.

